Our Platforms
Redefine Biomanufacturing
NTx is enabling access to tomorrow’s
therapeutics, today.
Accelerate your breakthroughs with NTx Bio’s continuous flow platforms. We eliminate the manufacturing bottlenecks inherent in conventional RNA and protein synthesis, putting high-quality manufacturing on any benchtop to enable faster development cycles and seamless scaling. Our technology provides the agility and efficiency needed to bring tomorrow’s therapies to reality, today.
How NTx is Revolutionizing Biomanufacturing
Conventional Batch Process Manufacturing
Cost $Bs to build a manufacturing plant
- Conventional plants built for volume
- Smaller liquid handling systems cannot deliver scale
Requires significant process development (days to months) and retrofit for each “batch”
- Static process
- Cannot be easily distributed or mobilized
NTx Continuous Flow Process Manufacturing
Cost
Small, low-cost footprint that delivers high yield, high integrity mRNA
Scalability
- Consistent process from discovery through clinic
- Linear scaling from personalized doses to public health response
Speed
Hours. Rapid set-up and minimal development
Agility
Fully-closed compact system can be deployed to any bench anywhere, enabling production at the point-of-care
Reinventing Biomanufacturing to Improve Care
SPEED
Bench-top turnkey automated mRNA system processed >50mg of purified high-quality mRNA in a day
SCALABILITY
Continuous flow HFBR offers superior scalability from milligram to gram scale mRNA synthesis
AGILITY
Small footprint enables rapid deployment in portable or mobile cleanrooms
QUALITY
Minimizes RNA product degradation and contamination associated with conventional batch manufacturing processes
NTxscribe® Process (IVT + Purification)
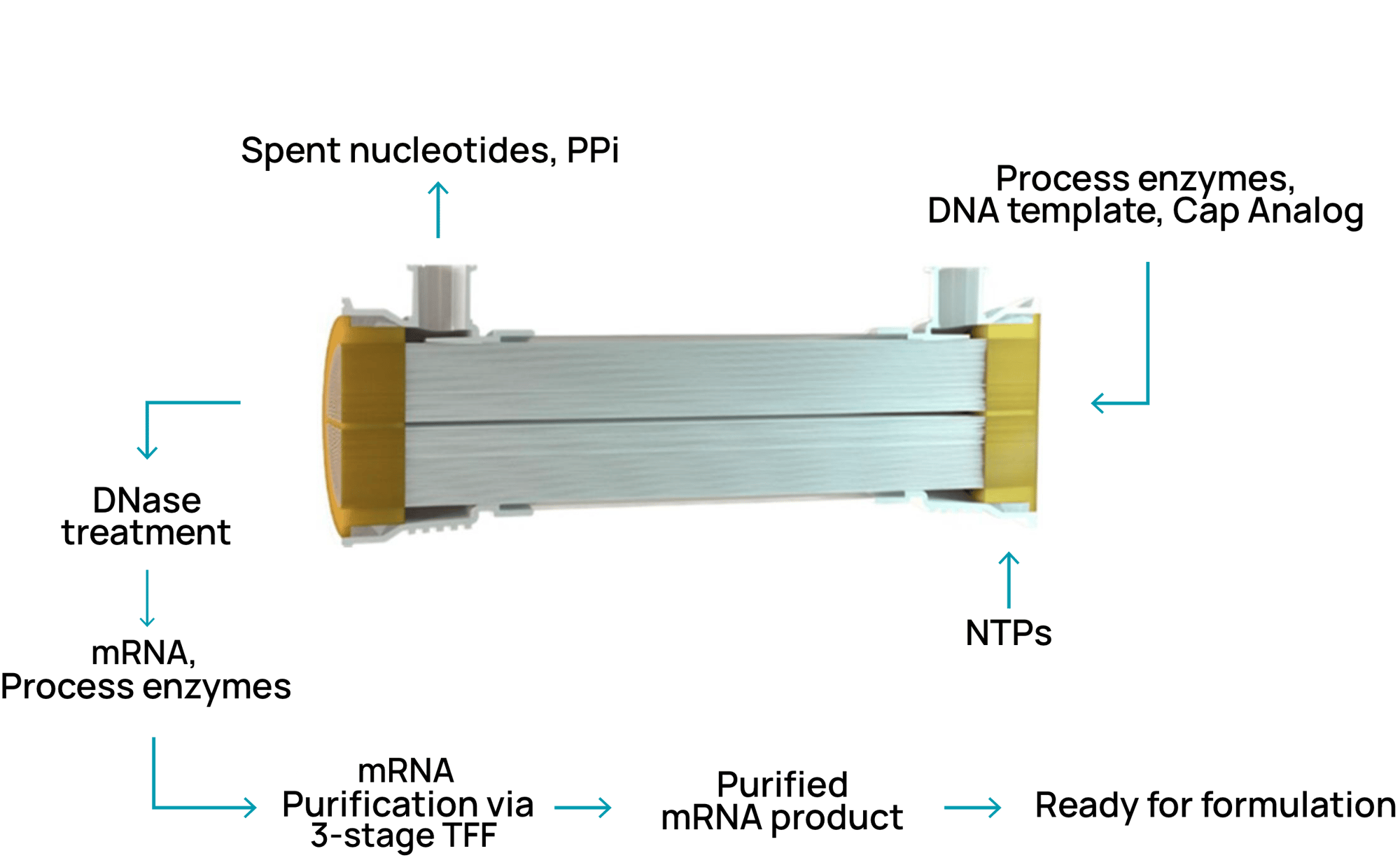
- Single-use, closed system configuration to ensure product sterility
- Decreased shear and handling, minimizing product degradation and contamination
- Streamlines manufacturing operations by reducing batch unit operations
- Enables linear scaling from mg to gram scale
- Benchtop modular design can accommodate different drug substance formats in small scalable footprint
- Allows for integration of in-line process analytics
